

Chapter 11: Climate Regulation and Atmosphere Evolution through Geologic Time
What do we mean by Climate?
Climate is not the same as weather. It basically
describes seasonal and daily
weather events over a long term period. For example, the passage of a cold front
over Arlington would be considered a weather event, whereas the daily average number of
such passages for the month of November (averaged over several years) is part of the
climate record. Climate records are usually expressed in terms of temperatures, wind
speeds and directions, precipitation, and pressures - all parameters that can be measured
at multiple sites around the globe. Over the years a large data base of weather event
measurements has been obtained, leading to a good description of today's climate. We also
find that climate varies widely around the globe - we have deserts and rain forests, ice
caps, temperate regions, etc. In the context of our course we are especially
interested with global climate: The overall conditions of temperature, circulation, and
humidity distribution for the planet as a whole.
Early Earth History: The Archean
(4.5 to 2.5 billion years)
After the Earth had developed a solid crust, as well as oceans, and an atmosphere due to volcanic outgassing of the mantle, we can reasonably assume that crust and atmosphere were close to chemical equilibrium. It is at that point that the famous primordial soup thickened and gave rise to replicating molecules (RNA world) and finally to primitive living structures. We know from an examination of early Archean rocks that there was free water in lakes and seas, and if life indeed formed as envisioned in the previous chapter the temperatures should have been somewhere between 0 degrees and 50 degrees Celsius (certainly above freezing and below the boiling point).
At this point the conditions on Earth were probably similar to this scenario:
This latter property will turn out to be a key ingredient for understanding the evolution of the Archean Earth. Also, because the internal heat production was about three times that of today (more radioactive material), we had more vigorous volcanism and a larger supply of volcanic gases.
What kept the Earth warm, however, was not heatflow through the crust, but solar energy (remember, solar input is several orders of magnitude larger than heat flow through the crust). Actually, considering that the heat output by the Archean sun was probably about 25% lower than today, we may wonder whether the surface temperatures were within the comfort zone for life as we know it. Under the assumptions in the above scenario of a carbon dioxide-rich atmosphere (10-40%) and a low luminosity sun, scientists have tried to estimate the surface temperature of the early Earth. The result of such calculations, taking into account the greenhouse effect, are surface temperatures around 20 to 25 degrees C, subtropical to tropical conditions. That this is reasonable is also indicated by observations of Archean sediments that suggest the presence of open bodies of water on the planet’s surface.
As suggested in the previous chapter, the initial life forms probably made use of the abundance of organic components in the oceans, and apparently some of them evolved to tap into solar energy through the use of photosynthesis (cycanobacteria or blue-green algae). Also at about this time must have occurred the evolution of methanogenic bacteria, bacteria that utilized the waste products and leftovers of the photosynthesizers for their metabolism and generated methane and carbon dioxide as a byproduct.
The invention of photosynthesis was a major step for organisms on Earth, and from then on organism took on a more important role. For one thing, the utilization of carbon dioxide as a raw material for synthesizing carbon compounds (more cyanobacteria), has a tendency to remove carbon dioxide from the atmosphere and to potentially lower the atmospheric CO2 levels (assuming some carbon burial in sediments). But that early in the game, lots of CO2 was essential for keeping the earth warm enough for living things. So, the invention of photosynthesis could have been the beginning of the end (use up too much CO2, temperature drops, oceans freezes). If there had only been cyanobacteria (assuming the covered most of the ocean surface), they might have been able to reduce atmospheric CO2 to dangerously low levels within a few million years, despite the continued input by volcanoes. Yet, the geologic record shows that this apparently did not happen. We have no record of ice ages that early in Earth history. James Lovelock suggests that it were the methanogens that saved the day. By breaking down the organic matter produced by cyanobacteria they return CO2 and methane to the atmosphere and thus amplify the greenhouse effect. In a way the methanogens are the analog to the black daisies (their activities increase global temperature), and the cycanobacteria are the analog to the white daisies (their actions reduce global temperature).
Another one of the consequences of photosynthesis was the release of free oxygen into the environment. Initially, all the oxygen that was produced immediately combined with reduced iron in the Archean oceans and gave rise to sediments with a high content of iron oxide (probably iron oxy-hydroxide initially, and later altered to hematite). The resulting sediments are called sedimentary iron formations and have a characteristic layered appearance (banded iron formation or BIF).
 |
Picture of Banded Iron Formation (BIF) from the Archean of Michigan. These sediments form entire mountain ranges in Michigan and have long been a main supply of iron ore for the US. |
 |
Iron oxide was also trapped on stromatolites and led to stromatolites with hematitic laminae. |
For about 1.2-1.3 billion years since the onset of massive photosynthesis, banded iron Formations were a very common marine sediment in the Archean. Sediments that were eroded from the land surface at that time contain detrital pyrite and uraninite, both minerals that would be destroyed during weathering in an oxygen bearing atmosphere. Thus the atmosphere was basically free of oxygen because all the oxygen that developed was buffered (consumed) by the dissolved iron in the oceans.
Things changed at about 2.5 billion years ago, when the dissolved iron buffer neared exhaustion and free oxygen gradually entered the atmosphere. This time marks the beginning of the decline of banded iron formations, with last occurrences dated at about 2 billion years. Yet, although the oceanic iron reservoir was being exhausted, there was yet another reservoir of reduced iron that for a while was able to keep atmospheric oxygen levels low. This reservoir was the mass of reduced minerals (mafic minerals with reduced iron, pyrite) that were available on the land surface. This iron is oxidized to iron oxide, mixes with fluvial sediments, gives them a reddish color, and causes them to be called "red beds". The oldest red beds are about 2 billion years old, and their appearance on the stage coincides with the disappearance of banded iron formations. Thus, prior to the buildup of significant noticeable quantities of oxygen in the atmosphere, the oceans were cleared of reduced metals.
One consequence of oxygen entering the atmosphere (even at minute levels) is the oxidation of methane and the lowering of methane to trace levels. This probably happened sometime between the final drawdown of the oceanic iron reservoir (about 2.5 billion years ago) and the onset of red bed formation (about 2 billion years ago). Because methane is a greenhouse gas, its essential disappearance (see below) also implies a drop in temperature. As it so happens, at this point in Earth history we have the first ice age.
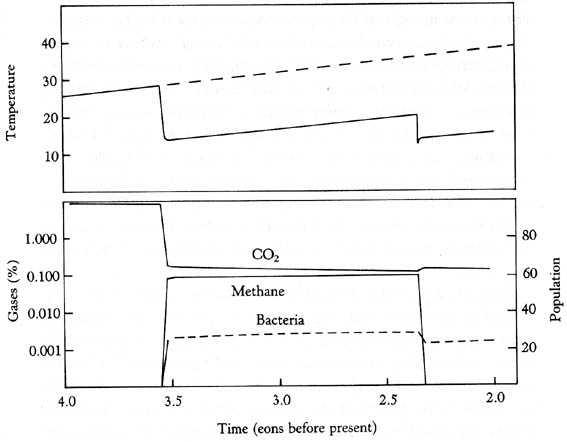
A model of Archean temperatures and atmosphere composition before and after life
evolved.
The model utilizes the action of photosynthesizers and methanogens in analogy to black and white daisies (see above). We see that temperatures drop with the onset of photosynthesis, that a balance between photosynthesizers (CO2 consumption) and methanogens is established with an overall lower equilibrium temperature. The next temperature drop occurs when methane drops due to oxygen buildup (the iron buffer fails). This point in evolution marks the end of the Archean and the beginning of the Proterozoic (link to time scale). In this model, once life starts it very quickly colonizes the available space (positive feedback mode), and then settles into homeostasis for the long run. When interruptions occur, like the change from reducing to an oxidizing atmosphere, the system reacts and moves to a new homeostatic level. Although there are a number of documented large meteorite impacts during the Archean, impacts that conceivable could have wiped out half of planetary life, these interruptions or catastrophes were not sufficient to crash the system. If we consider planetary self-regulation a la Daisyworld a distinct possibility, the fact that life is still here and prospers is testament to the inherent stability of the system.
| GAS | Before Life | With Life |
| Carbon Dioxide (CO2) | dominant | 0.3% |
| Nitrogen (N2) | unknown | 99% |
| Oxygen (O2) | absent | 1ppm |
| Methane (CH4) | absent | 100ppm |
| Hydrogen (H2) | some | 1ppm |
Studies of Archean sediments suggest that high atmospheric carbon dioxide levels did not persist long into the Archean. Because high concentrations of CO2 imply very acidic rain and surface waters, we should see very high rates of weathering in the Archean if they had stayed high. Because we do not see this effect we must assume that atmospheric carbon dioxide levels were quite low (probably below 1%). The CO2 levels indicated in our model are in that range, and suggest (but do not prove) that the Archean geologic record is consistent with an atmosphere that was compositionally modulated by the interplay of photosynthetic and methanogenic bacteria.
The production of oxygen had another, possibly very important, effect on the long term health of our living planet. This is so because chemical reactions between water and reduced iron and sulfur in surface rocks cause the release of hydrogen gas, and that gas is then able to escape from the earth atmosphere (velocity of hydrogen molecules large enough to exceed escape velocity). Escape of hydrogen has one important consequence, it robs the planet of water -- escape of an H2 molecule means that a molecule of water is forever lost. Mass balance calculations for the early Earth suggest that, had it not been for life (photosynthetic bacteria), the Earth would have dried up about 1.5 billion years into the Archean because of hydrogen escape (this may be what happened to Venus).
There are several ways in which the Archean biosphere may have helped to conserve hydrogen:
In the past, scientists thought that oxygen was a vital component of the atmosphere, and that life at the surface of the oceans and the continents could not have existed had it not been for oxygen in the atmosphere. The underlying reason is that today, the ozone layer (O3) is protecting the earth's surface from harmful ultraviolet radiation. The sterilizing effect of UV radiation was thought to have killed off all life that had ventured into shallow water and on the land surfaces. Yet, UV- based sterilization methods fail when microorganisms are covered with organic mucus secretions. Because this is the way most microbes exist in the real world, the sterilizing effects of UV radiation have been overstated as far as early life history is concerned. As we see from our considerations to this point, there could not have been any ozone at the beginning of life, because any free oxygen would have immediately reacted to form other compounds, and life certainly did not hesitate to colonize the shores and tidal flats of the young Earth (stromatolites, microbial mats, etc.).
Within
Archean sedimentary sequences we can find black shale units that are quite high
in organic carbon (I have seen examples with as much as 10%). This carbon (or
graphite when found in metamorphosed rocks) is all what remains of microorganisms that
thrived in the Archean ocean, and its burial and preservation in marine sediments
indicates that the "biological pump" was active as far back as the
Archean.
As we know from chapter 8, the "biological
pump" is an essential component of the global carbon cycle, in that it removes
carbon from the atmosphere-ocean system and thus lowers atmospheric CO2 levels and
increases the amount of oxygen kept in the atmosphere.
This suggests that basically
the same system of climate and atmosphere regulation was already active in the
Archean,
and that today we simply exist at different homeostatic levels for the various atmospheric
gases.
The Middle Ages of Earth: The Proterozoic (2.5 billion to 570 million years)
The Proterozoic is basically the time span between the appearance of free oxygen in the atmosphere and the earliest record of body fossils. With body fossils we mean organisms that had skeletal hard parts (the shells of trilobites, clams, and snails for example), that were preserved as fossils in the rock record. Like the Archean, the Proterozoic was for most of its duration dominated by bacteria. Many surface sediments (marine and lacustrine) were anoxic because the oxygen was consumed by bacterially mediated decay processes, and these were the places where Archean bacteria lived on (their descendants probably are still around in modern anoxic sediments). The shallow water and surface environments were mildly oxidizing now because the oceanic iron buffer had been exhausted, and because the combination between photosynthesis and carbon burial had allowed the presence of free oxygen in the atmosphere and in ocean waters. In essence the living world had been split into two coexisting ecosystems, the oxic surface regions and the anoxic sediments. In a way the Archean did not end, it simply went underground.
As the Archean world changed into the Proterozoic one, shifts occurred in the atmosphere, the oceans, and in the world climate. Although direct evidence is lacking on whether changes in atmospheric composition were abrupt, or whether they were gradual or occurred in steps, there is one feature in the geologic record that suggest a comparatively sudden change. At about 2.3 billion years before present we had a major ice age (the first one in recorded Earth history). The cause for this ice age could for example be explained by a drop in methane content. Methane and oxygen will combine spontaneously in the atmosphere (reactions induced by sunlight) to form water and carbon dioxide, and because an exhaustion of the iron buffer allows oxygen levels to rise, a side-effect could have been a lowering of methane levels. Methane (as well as its decomposition products) is a greenhouse gas, and its removal, one would surmise, should have caused a lowering of global temperatures. Also, more efficient oxygen using consumers could have utilized organic matter in the water column, before it ever had a chance to sink to the bottom to supply the methanogens with food. Thus, the rise of atmospheric oxygen could have impeded methane production and preservation at several levels, and the effects should have grown stronger with rising oxygen levels (positive feedback).
While oxygen was probably a crucial element in the evolution of the atmosphere, calcium may have been a most important element for the changes occurring in the oceans and in the crust. After sodium and magnesium, it is the third most abundant positive ion (cation) in seawater, and we have seen earlier (chapter 8) that calcium is connected to the carbon cycle via weathering of the crust and precipitation of carbonate minerals. Calcium is essential for life (think of the many calcium fortified foods), but paradoxically, it is quite toxic in the ionic state. If the calcium concentration in our cells exceeds a few parts per million it will kill us (as well as other organisms). In the ocean however, calcium levels are four orders of magnitude (10,000 times) higher. In light of this it is tempting to think that organisms that developed the capability to precipitate calcium carbonate would have done themselves a favor by reducing the potential toxicity of their environment, and by getting rid of the calcium that tries to invade their cells via diffusion. Now, this is not a view that is universally shared by geologists, and there is undoubtedly opportunity for carbonate precipitation in the oceans without the direct involvement of organisms. Nonetheless, just as today, it seems that the bulk of Proterozoic (and Archean) carbonate precipitation is tied in one way or other to organic activity. Microbial mats, for example in the form of stromatolites, seem to be involved in most of the carbonate production during the Archean and Proterozoic. Recent research suggests that even carbonate particles that were long thought to be inorganic precipitates form by biological mediation.
The fact that we have large deposits of stromatolitic and otherwise microbially produced carbonate in the Proterozoic (this started already in the Archean), attests to the fact that this process took large quantities of calcium ions out of the ocean waters and thus reduced the toxicity of the oceans for its living inhabitants. Whether organisms simply provided nucleation sites for calcium carbonate precipitation that would have occurred any way due to supersaturation, or whether their presence promoted a lower calcium ion level is still being debated, but the intimate association between microorganisms and calcium carbonate precipitation is certainly intriguing. One could argue that once some organisms got into the business of calcium carbonate precipitation by chance, the resulting lower calcium ion levels may have granted these organisms a better chance of survival and procreation, and thus giving them an evolutionary advantage over those organisms that merely manage to tolerate higher Ca levels.
Salt regulation is also a question that we have to consider when talking about the early Earth. Above about 6% salinity, most living organisms, even the hardy bacteria, get pickled and die. So, because the oceans constantly receive dissolved salts from the continents, the question needs to be asked whether the oceans were kept below this limit simply by chance, or whether coupling of the evolution of life and the environment led to self-regulation of ocean salinity. On the other hand, by looking at the solubility products of oceanic salts, one can provide a chemical argument by which ocean salinity should most likely never have been higher than twice the present level.
How does salt removal work in practice? What we see throughout the geologic record is this: along the margins of continents lagoons form, often the byproduct of organic buildups, such as coral reefs (or stromatolite reefs in the Precambrian). Because these organically build barriers separate the lagoon from the open ocean, if it is hot enough water will evaporate, form layers of salt, and these can then be buried beneath lagoonal sediments and taken out of the system. In modern lagoons where this happens, blue-green algal mats often cover the salt layers and prevent their dissolution during intermittent flooding of the lagoons. Some geologists even speculate that the buildup of large quantities of biogenic carbonate and evaporite deposits along the continental margins may have been a factor in the initiation of plate tectonics on Earth. So, we have here tantalizing glimpses of how organisms might be involved in salt regulation, recycling of soluble elements, and may be even plate tectonics, but how this might have worked is everyone's guess. The steps from the individual lowering of calcium ion concentrations within the cells of living organisms to the movement of plates and crustal recycling all have a tendency to improve the environment of the organisms responsible. But the links between biomineralization, salt stress, an plate tectonics are so tenuous, that we have to conclude that we still know too little about the biogeochemical cycling of elements to make even qualitative statements in this regard.
From Prokaryotes to Eukaryotes
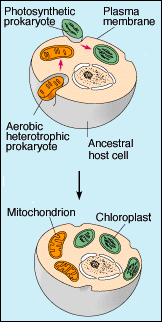 How early cells might have added new capabilities by merging in symbiosis with other cells that had evolved advantageous capabilities. |
During the Proterozoic, a new type of cell evolved. In contrast to the bacterial (prokaryotic) cells, these new cells (eukaryotes) have a nucleus and other new cell-internal structures (organelles). These more complex cells probably evolved as communities of bacteria that now are contained in a single cell membrane, the so-called endosymbiotic theory, pioneered by Lynn Margulis. The endosymbiosis theory suggests an explanation for the existence of certain cellular structures in eukaryotic cells. According to the theory, complex eukaryotic cells evolved from the symbiotic relationship (living together) between larger cells and smaller cells that started as either undigested meals or parasites of the larger cells. Endosymbiosis (living together inside) is not uncommon in nature; however, this theory suggests that mitochondria (the powerplants of eukaryotic cells) and chloroplasts (the photosynthetis plant) originated as free-living organisms, and that modern eukaryotic cells are essentially communities of different cell types living cooperatively. |
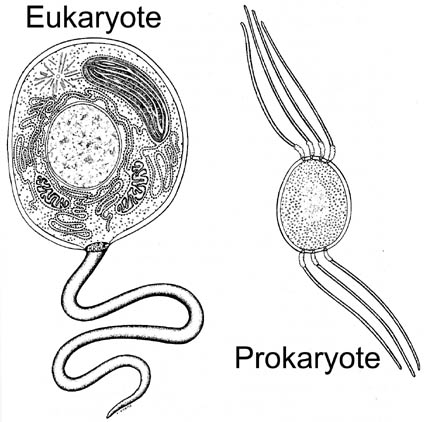 |
A comparison of eukaryotic and prokaryotic cells. The eukaryotes differ in having membrane-bound cell organelles which includes the nucleus (storage of genes), mitochondria, and chloroplasts. |
 |
The first fossil eukaryotes go back to about 1.8 billion years. Some examples that are preserved in cherts may even show remains of a nucleus (may be an artifact of cell decay) |
 |
A large number of the Proterozoic eukaryotes were seaweeds and acritarchs, the spherical fossils of likely algal protists. |
More on Proterozoic Oxygen
In principle, free oxygen can come from two sources: (1) the escape of hydrogen to space, and (2) the burial of reduced carbon (organic matter) or sulfur (pyrite). Once free oxygen makes its debut, the escape of hydrogen becomes negligible, and the further loss of hydrogen (and indirectly of water) is stopped (or so small as to be of no consequence). From then on, to increase oxygen it has to be split off CO2 or SO4, and the resulting reduced carbon (organic matter) or sulfur (H2S or S) has to be buried and put into the crustal reservoir. Most of the carbon that is split off during photosynthesis is recombined with oxygen during decay processes, but a small portion (about 0.1% today) is buried for long time periods in the rock record. Use of carbon by bacteria to split off oxygen from seawater sulfate leads to burial of pyrite (H2S) and means another net gain of free oxygen.
A question that comes to mind in this context is whether burial of pyrite in sediments will directly cause atmospheric oxygen to rise? Yes, burial of pyrite implies that sulfate reducing microbes split oxygen from the sulfur of the SO4 ion dissolved in seawater, but in contrast to plants that simply release O2 into the environment, the sulfate reducers use the oxygen to combine it with organic carbon for energy gain, making CO2. The sulfur is released into the environment as H2S and combines with iron to form pyrite (which is them buried and removed). Thus, sulfate reduction in itself does not directly raise atmospheric oxygen levels. Only once the produced CO2 is picked up by photosynthesizers will we see the formation of free oxygen.
Overall therefore, the amount of carbon that is buried determines the amount of free oxygen production and atmospheric oxygen levels. So, how would our Earth system set a homeostatic oxygen level?
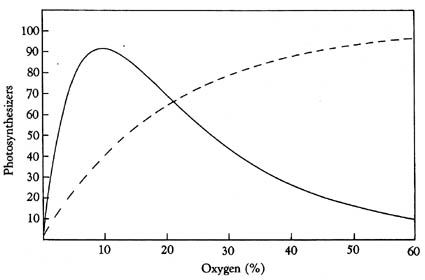 |
The influence of oxygen levels on the growth of organisms in a modern oxic ecosystem
might be instructive here. The effect of oxygen on the growth of organisms (solid line), and the effect of the presence of organisms on the abundance of oxygen (dashed line). The solid line is the relationship between a steady level of oxygen and the population of oxygen-using consumers. At low levels of oxygen they don't thrive because they can not metabolize, and when oxygen levels are to high they are "poisoned". The dashed line shows the relationship between the population of the oxic ecosystem (photosynthesizers) and the oxygen level of the environment. The more photosynthesizers, the more oxygen. Where the two curves intersect is the level of oxygen at which the system is regulated. |
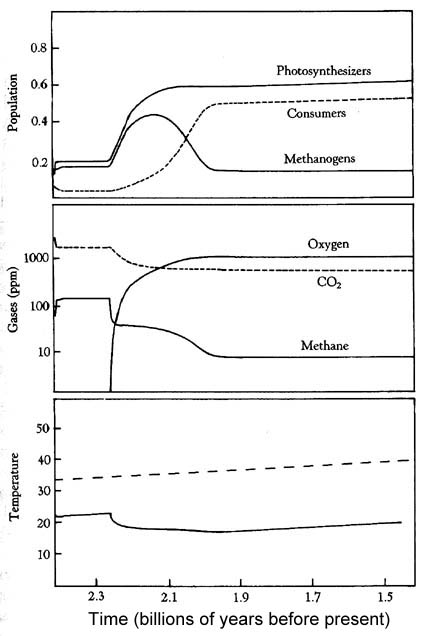 |
A model of the change from the Archean to the Proterozoic. In the model photosythesizers (producers), consumers, and anaerobes (methanogens), coexist. It is assumed that through a constant burial of carbon and a declining turnover of reducing rocks and gases, the planet is gradually oxidized until free oxygen appears. The upper panel shows population changes of the ecosystem. |
| The middle panel shows the abundance of atmospheric gases. As atmospheric oxygen levels rise, increase of weathering and nutrient supply should help to support a larger ecosystem (larger biomass). As a result more carbon would be buried until O2 levels would limit further expansion due to toxicity. A new carbon/oxygen homeostasis is established. Increased weathering would also limit oxygen buildup. The size of the methanogen ecosystem would shrink, reducing methane levels. | |
| The lower panel shows climate (temperature) in a lifeless world (dashed line) vs a world where life has evolved (solid line). |
Because of the coupling of the oxygen and carbon dioxide cycles, as oxygen rises, carbon dioxide falls. Through the link with the greenhouse effect and climate, the result of all this is also a lowering of temperature. In general we have two feedback components: (1) as temperatures drop, producers and consumers are negatively affected and their population drops. The resulting reduction of CO2 drawdown and continued CO2 production by volcanoes would lead to a gradual increase in CO2 again, and a return to higher temperatures. (2) as temperatures rise, conditions improve and result in a growing population (biomass). More and more CO2 is utilized and with a constant fraction of the production being buried, we raise O2 and decrease CO2 levels. That in turn leads to a reduction of the greenhouse effect, temperatures begin to drop, and we go back to (1). Once the initial oxygen "crisis" at the beginning of the Proterozoic was overcome (we had our first ice age), climate settled down to very comfortable conditions for Proterozoic life.
At the beginning of the Proterozoic the sun was cooler than today, and the greenhouse effect (and CO2) were needed to keep the Earth from freezing. While things were comfortable overall, a number of large meteorites hit the Earth during the Proterozoic, and some of them might have been large enough to wipe out as much as 60% of surface life. Yet, despite these setbacks life persisted and the climate was held in check. One could take this observation as an indication of a powerful homeostatic control system for the Earth climate.
Recent Earth History: The Phanerozoic (570 million years to present)
 |
Towards the end of the Proterozoic another biological revolution occurred, the evolution of multi-celled organisms that may have constituted the first animals. These early animals had no hard parts, and thus left only impressions in the rock record. This latest time period of the Proterozoic where these creatures are found is called the Vendian, and the fossils have become known as Ediacaran Fauna, after the place of their first discovery in Australia. Research into the systematics of Ediacaran fossils is still in flux, but the pictures in the Ediacaran link show some examples. |
At the beginning of the Phanerozoic multicelled animals underwent a dramatic "explosion" in diversity, and almost all living animal phyla appeared within a few millions of years. The new thing about these fossils is that they are usually large enough to be seen unassisted by microscopes, and that they have hard parts that are more readily preserved in the rocks record (as compared to the impression of soft-bodied animals). Probably the most famous of these early animals are trilobites, an early kind of arthropod (lobsters, crabs, and insects are arthropods). The arrival of these new inhabitants changed the pace of life on Earth and was apparently again accompanied by a change in atmospheric oxygen content.
Up to this point, there has been an interdependence of producers (photosynthesizers) and consumers in the Earth system. Without consumers, the producers would exhaust the CO2 store of the atmosphere quite rapidly, and this would not only reduce their ability to thrive, but might also cause surface temperatures to drop beneath tolerance levels. This relationship continues throughout the Phanerozoic. Plants develop ever more intricate ways to keep the consumers at bay (poison, spines, bark, etc.), and the consumers constantly adapt to overcome these obstacles.
As discussed above, during the Proterozoic, cyanobacteria had transformed the Archean ocean-atmosphere system from an anoxic to a mildly oxidizing system. The increase in O2 increased the weathering of the continental crust and increased the supply of nutrients, salt and calcium into the ocean. The rising levels of these nutrients led to a larger biosphere where cycanobacteria and planktonic eukaryotes extracted more CO2 from the atmosphere, buried more carbon, and therefore produced more O2. This O2-producing positive feedback was balanced by 4 factors:
Because of the small size and thin walls of
Proterozoic organisms, the optimum O2
levels for these unicellular organisms were probably only 0.1% of the present level.
Oxygen levels much higher than this would have been toxic, especially for the simple
eukaryotic cells that oxidized their food during respiration. This is because respiration
produces highly reactive oxygen radicals such as hydroxl ions (OH), hydrogen peroxide,
superoxide ions and normal O ions. To cope with these toxic waste products eukaryotes and
cyanobacteria had to develop antioxidants that neutralized such wastes in order to
detoxify their cells (enzymes such as catalase, dismutase, etc. that decompose these
radicals into harmless products). For these reasons
oxygen concentrations in the
Proterozoic ocean-atmosphere system remained low and consequently prevented the
development of larger multicellular eukaryotes who, like modern organisms, require O2
levels that are at least 10% of present levels.
At the latest by about 600-550 million years before present, oxygen levels must have risen
dramatically and must have reached the 10% threshold. One reason why scientists believe
that oxygen levels increased at the changeover from Proterozoic to Phanerozoic is because
of the kinds of new
lifeforms that appeared. The modern descendents of many of these
lifeforms require oxygen levels in their environment that are substantially above the
(still) low levels that were typical for most of the Proterozoic (Proterozoic oxygen
levels were probably below 1% for most of the time). Small organisms, such as the
the many single celled ones of the Proterozoic could make do with low environmental oxygen
concentrations. Their large surface to volume ratio allowed for sufficient oxygen
supply by diffusion. Larger creatures, especially animals, need to be able to supply
oxygen to their body interior, and a high ambient oxygen supply is very advantageous in
that regard. Because of the toxic effects of oxygen, too much of a good thing can be bad
in the long run. Thus, too little oxygen is not good for animals, too much, on the
other hand, can do damage to the organism. These new lifeforms, large multicellular
animals collectively described as metazoans, showed striking similarities to some modern
metazoans including jellyfish (cnidaria), sea-anemonies and sea pens (anthozoa), and
segmented worms (annelids). They were introduced earlier as the so called Ediacaran fauna. Then as quickly as
they had appeared, the Ediacarans disappeared without a trace. Why this happened is still
unclear, but two possible causes are: (1) fluctuation or instability in the oxygen levels
of the late Proterozoic; or (2) biological pressure from the evolution of competitors,
consumers or scavengers.
Whatever the cause of the Ediacaran demise, it wasn't long before the more advanced
metazoans abruptly reappeared, and this time they were fully equipped for competition with
lethal weapons and body armor that are the hallmark of complex modern predator-prey
ecosystems. This abrupt entrance of complex ecosystems has been called the Cambrian
Evolutionary Explosion of life, and it is recognized by the appearance of mineralized
endo- and
exo-skeletons in rocks that are 540 Ma or younger.
This change marks the
beginning of the Phanerozic -- the Era of "visible life" -- when nearly all
classes of life, that now exist on the earth, appeared. Other metazoans, however,
remained naked and vulnerable and protected themselves by living, burrowing and feeding
beneath the sediment. In fact they developed quite sophisticated behavioral strategies
that burrowing organisms still use today.
Oxygen and the Cambrian Explosion
The big question is why did the Cambrian Explosion occur? The degree of sophistication in
body plan and ecosystem complexity clearly showed that the
oxygen level had now stabilized
at optimum levels for life between 10-25% -- in other words very similar to today's
levels. So the problem then becomes what mechanisms allowed oxygen to build up to
modern levels and how have these levels been maintained for the last 540 Ma?
Two
hypotheses that have been advanced to explain the Cambrian explosion and the build-up of
oxygen are (1) plate tectonic control; and (2) a biological control.
In the first hypothesis it is proposed that during the middle and late Proterozoic, plate
tectonic convergence had gradually amalgamated pieces of continental crust into one
large
supercontinent called
Rodinia (russian for motherland). This supercontinent
began to rift
and break up 700 million years ago, (about 200 million years after limestones started to
record carbon 13 enrichment and rising O2 levels, but only 100 million years before the
rise of the first metazoans in the Ediacaran fauna).
The idea is that during this initial rifting stage,
narrow ocean basins developed that had
poor circulation and periodically became stratified with anoxic waters at the bottom and oxic waters at the top. This allowed
high productivity of marine algae at the surface.
When these algae then sank into anoxic waters, they underwent partial decomposition
by anaerobes and eventually were buried in large volumes. This loss of carbon from the
ocean-atmosphere system produced a rapid rise in the proportion of free oxygen. This
process was episodic because, just like modern restricted seas like the black sea,
stratified water bodies commonly overturn causing nutrient-rich anoxic bottom waters to
mix with surface water. This causes intense blooms and burial of large amounts of organic
carbon. Other possible consequences of the rifting of Rodinia would be the sudden
creation of large volumes of fresh basaltic crust at the spreading ridges. This, it is
argued, might have changed ocean chemistry, raising the proportion of Ca ions to Mg
ions and therefore favoring the precipitation of calcite, as organisms tried to detoxify
their cells, and rapid evolution of new shelly faunas.
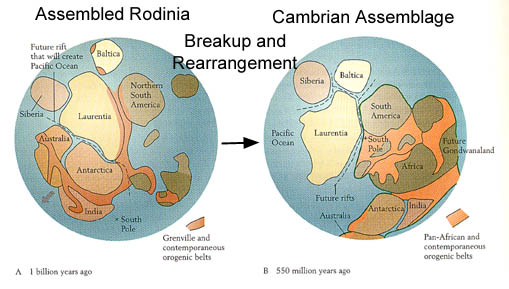
The supercontinent Rodinia, and its breakup and possible rearrangement by the
early Phanerozoic.
But there are problems with this rifting idea. New ocean crust produced at the spreading
ridges would have used up more oxygen as a result of
(1) oxidation of reducing elements in
the new basaltic crust and (2) because of oxidation of
sulphuric elements in
volcanic gases that would accompany plate tectonic activity. Another problem is that as
the rifting ocean basins got wider, they would have eventually developed better
circulation and the high rate of carbon burial would cease and O2 level would fall. In
other words, although the rifting
Rodinia hypothesis can produce instability in oxygen
level it could not have sustained it for long enough to maintain the explosion of life. So there must have been another more permanent
mechanism for burying more carbon and producing more oxygen.
A good candidate for a biological mechanisms that could have led to more carbon burial is
the invention of lignin and humic acids. Both of these substances are
biopolymers
that are very stable and highly resistant to biodegradation. They were probably first
produced as a mechanisms to detoxify cells of oxygen radicals. For example, in
modern plants phenols react with hydroxl (OH) radicals to produce lignins. Basically,
lignin synthesis in cells serves a similar function as for example calcite precipitation.
Lignin synthesis detoxifies cells of oxygen radicals, just like the precipitation
of calcite detoxifies cells of Ca2+ ions. The
resistance of
lignins and related substances
could have led to more carbon burial (the decomposers would only succeed to return a small
fraction of that material to the atmosphere), and to a
rise in O2 levels. Both
calcite and lignins provide the structural components for animal and plants respectively,
and produce large metazoan body plans. Evolutionary advantages associated with production of
these materials would be that by using them as skeletal materials, the organism would
simultaneously achieve (1) tissue detoxification, (2) protection from predators (external
armor etc.), (3) the chance to increase size, (4) and the development of better
locomotion.
O2 levels for the past 550 million years
Although there is no way to exactly measure the O2 content of the ocean-atmosphere system during the Phanerozoic, there is evidence to suggest that O2 levels have remained stable enough over that time period to allow metazoans to evolve and diversify into the great numbers of organisms we see today. Considerations of the flammability of land plants suggest that the oxygen content must have been pretty close to the present level for at least the last 350 million years. This is so, because below an O2 level of 15%, even dry twigs will not burn, and that above 25% O2, fires would be so fierce as to consume even the damp tropical rainforests. In the geological record we find charcoal from wood fires for the last 350 million years, an indication that O2 levels were between 15-25 percent. Our current level of 21% is about halfway between these extremes. However, recent experiments to test this hypothesis suggest that at O2 levels between 20-35% there should have been no sustained burning of forests as long as they were of normal moisture levels for live plants (article in GEOLOGY, May 2004, p. 457-460). Thus the "self-regulation by forest fires" hypothesis may not work, and we have to look into alternative regulation mechanisms.
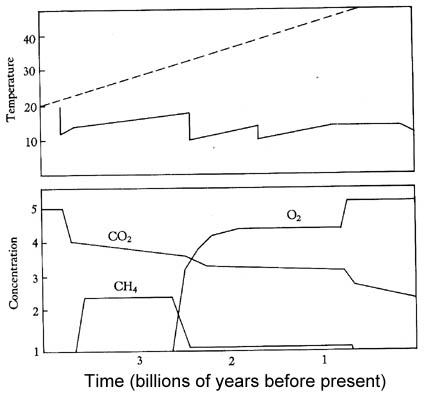 |
Computer models by Lovelock suggest that
throughout Earth history there are long
periods of stability or homeostasis that are punctuated by large changes in atmospheric
levels of CO2, O2, and Methane. In the Archean, when the sun was cool, bacteria kept CO2 high and stopped the earth from freezing. In the Proterozoic the sun was heating up and organisms didn't have to remove much CO2 to keep conditions just right for life, but they added considerable amounts of oxygen to the atmosphere-ocean system. In the Phanerozoic, the sun gradually became hotter, so the organisms had to remove more CO2 to prevent the earth system from overheating, and as a result more O2 was added to the atmosphere. Evolution of metazoans may simply be an opportunistic response to the new possibilities that opened up with higher O2 levels. In many ways, oxygen plays an instrumental role when we look at the long term interaction between biosphere and Earth climate. |
Attempts to estimate the Oxygen history of the Phanerozoic Atmosphere: With approximations of the various carbon fluxes discussed in chapter 8, derived in large part from the 13C isotope record of DIC (dissolved inorganic carbon) in seawater as recorded by sedimentary carbonates through geologic time, scientists try to reconstruct the sources and sinks of atmospheric CO2 and O2 for the past ~600 million years. Shown below is one estimate by Berner and co-authors (Berner, R.A., Petsch, S.T., Beerling, D.J., Popp, B.N., Lane, R.S., Laws, E.A., Westley, M.B., Cassar, N. Woodward, F.I., and Quick, W.P. (2000) Isotope fractionation and atmospheric oxygen: implications for Phanerozoic O2 Evolution. Science, 287, 1630-1633.) for Phanerozoic O2 variations. We see that although more or less steady around 20% for most of the Phanerozoic, O2 had a minimum early in the Paleozoic and a strong maximum in the Late Paleozoic associated with significant burial of organic matter in Carboniferous sediments.
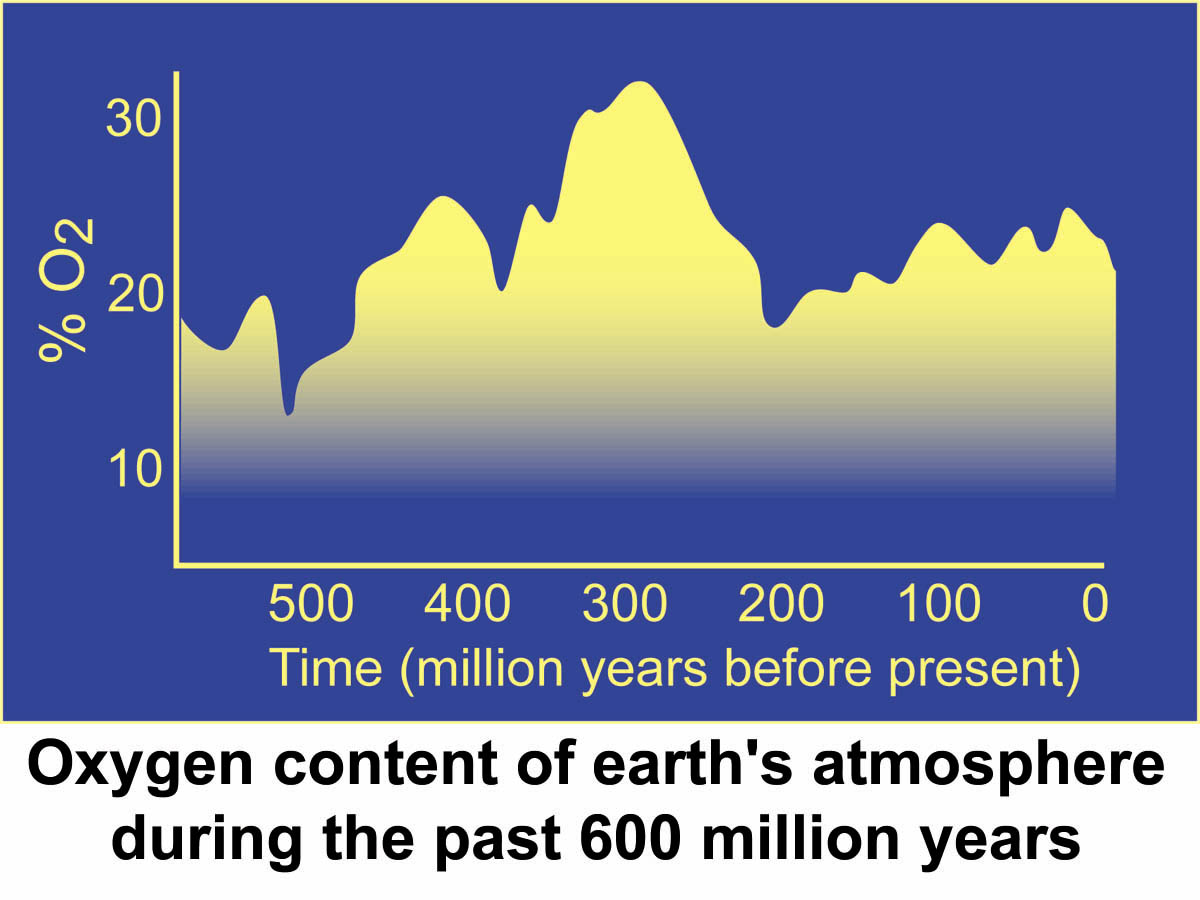
Evidence that oxygen levels may have
been unusually high comes from the fossil record. For example dragonflies
with wingspans of 2-3 feet were roaming the Carboniferous forests. And other
insects as well were much larger than today.
Considering the primitive way by which
insects absorb oxygen (no lungs), this demands higher O2 levels in the
atmosphere to allow these large versions to be mobile.

Below, images of a Carboniferous millipede (6 feet long!), and of a sea scorpion (6-7 feet long). Roaches up to 1 foot long are known from that time as well.


Other Ways of Climate Regulation: the Dimethyl Sulfide Story, or How Microbes Make Clouds
Another factor in climate regulation are clouds and the energy transfer involved in phase changes of water from gas to liquid and back. Typically, the air over the oceans is supersaturated with water, but clouds are often absent because supersaturation alone often does not initiate condensation. What is needed are condensation nuclei that allow water to form small droplets. Modern rainmakers have for example injected silver iodine and dry ice into moist air masses to stimulate cloud formation. It has for example suggested that modern algae produce sulfur compounds that are responsible for cloud formation.
Marine algae emit huge quantities of dimethyl sulfide to the air, and it has been proposed by Lovelock and others that rapid oxidation of dimethyl sulfide to sulfuric acid (aggregating into small droplets) could be a source for cloud condensation nuclei.
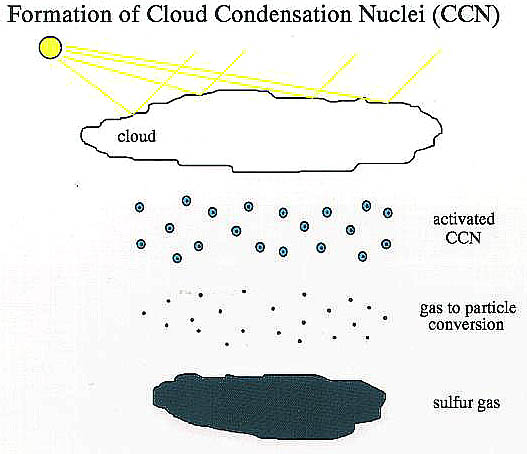
Micro-droplets of sulfuric acid are very efficient cloud nucleation nuclei. If they are due to algal production of dimethyl sulfide in the oceans, we may have yet another biologically mediated mechanism of climate regulation. Considering that from outer space, the color of the oceans is a dark blue, and that clouds are white, it is easy to see that there is a great contrast in albedo between ocean areas (low albedo) and cloud covered areas (high albedo). Lovelock and collaborators estimate that the climate effects due to dimethyl sulfide cloud nucleation is comparable in magnitude to that of the carbon dioxide greenhouse effect.
Marine production of dimethyl sulfide (a gas) is probably the main route in which sulfur (an essential nutrient) is returned from the oceans to the land. How marine algae evolved this capability and why is a matter of speculation, but it may have to do with the production of dimethylsulfonio propionate, a substance that allows algae to withstand extremes of salinity. That production of dimethyl sulfide by marine algae indeed is linked to cloud formation is suggested by close matches in dimethyl sulfide and cloud formation in the world oceans. Increased production of dimethyl sulfide could increase the cloud cover and the overall albedo of Earth, and could in that way promote a cooling of the planet. This capability might come in handy in about 100 million years, when scientists estimate that temperature regulation via the carbon dioxide greenhouse effect will fail (the Earth will overheat even at 0% CO2 because of increased solar radiation). By producing more clouds the algae could keep the oceans (and Earth) from boiling.
Comparing possible Atmosphere Evolution on Earth, Mars, and Venus
We have assumed that atmospheres accumulate gradually on initially cold and airless
planets. When there was no atmosphere, the temperature of the surface was given by the radiative equilibrium temperature, determined by the balance between incoming and outgoing
radiation. As the atmosphere accumulated, however, the greenhouse effect of water vapor
and also carbon dioxide began to grow, heating the surface. What happens next shows the
important role of water and its ability to exist in gas, liquid and solid (ice) phases for
different conditions of temperature and pressure.
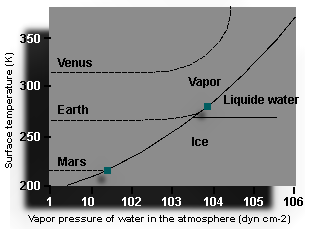 |
The figure at the left shows a schematic view of the role of the growing greenhouse effect on the early Earth, Mars, and Venus. Time runs from left to right as the atmosphere grows (increasing atmospheric pressure). The surface temperature begins to increase when enough water vapor has accumulated to absorb a substantial fraction of the infra-red radiation emitted by the ground. This condition is met when the rising vapor pressure for water is about 103 dynes cm-2. |
| The diagonal curve that goes from the lower left to the upper right
separates the stability field of water vapor from the stability fields for ice (lower
right) and liquid water (upper right). Lines that start out horizontally at the left
are the assumed surface temperatures (from radiation balance) of Mars, Earth, and Venus.
As can be seen for Earth, when the water vapor pressure reached a value of about
104 dynes cm-2, the conditions of temperature and pressure were such as to allow water to
condense into the liquid phase - this marked the onset of ocean formation. Continued
condensation limited the amount of water vapor in the atmosphere and therefore the surface
temperature (it was prevented from rising further; cloud formation also provides another
negative feedback). Once water condensed, the carbon dioxide begins to dissolve in the
oceans, and ultimately the carbon became buried in ocean sediments, leaving molecular
nitrogen as the dominant atmospheric constituent. The situation was different for Mars and Venus. Mars is further from the Sun and so would have started with a colder surface temperature. As the water vapor pressure increased, frost would have started to accumulate before any greenhouse effect could take place. Carbon dioxide would also have frozen in the polar region, forming the carbon dioxide polar ice caps that exist on Mars today. Under this scenario, life may never have evolved on Mars. Venus on the other hand is closer to the Sun than the Earth is, and would have started at a hotter temperature. The greenhouse warming effect of water vapor would never have been interrupted by a change in phase to either ice or water. So oceans would not have condensed on Venus, and outgassing (more and more CO2) would have led to an ever increasing greenhouse effect causing higher and higher surface temperatures. Such high temperatures keep water vapor in the gaseous phase (no chance for oceans, marine life, carbon burial, and CO2 lowering). This process is called a runaway greenhouse. Some thoughts about life in other parts of the solar system/universe. |
|
Background Music: The genetic code and music have many things in common, both are rules according to which nucleotide bases or notes follow each other. By assigning a tonal value to nucleotide bases or short sequences thereof one can transcribe genetic data into music. The background music is from the web page of the Nucleic Acid Data Base Project at Rutgers University.