High resolution image and legend (53k)
| Nature 417, 244 (2002), 16 May 2002 |
Phylogeny: A non-hyperthermophilic ancestor for Bacteria
The first phyla that emerge in the tree of life based on ribosomal RNA (rRNA) sequences are hyperthermophilic, which led to the hypothesis that the universal ancestor, and possibly the original living organism, was hyperthermophilic1. Here we reanalyse the bacterial phylogeny based on rRNA using a more reliable approach, and find that hyperthermophilic bacteria (such as Aquificales and Thermotogales) do not emerge first, suggesting that the Bacteria had a non-hyperthermophilic ancestor. It seems that Planctomycetales, a phylum with numerous peculiarities, could be the first emerging bacterial group.
rRNA is a useful tool for investigating the universal phylogeny of life, particularly for uncultured organisms2. The phylogeny of Bacteria based on rRNA is congruent with genomic data, such as phylogenetic trees based on multiple genes3, 4. This suggests that rRNA is rarely transferred and indicates that species phylogeny can be deduced by using this marker.
However, rRNA phylogenies can be seriously affected by artefacts of tree construction5. As the most slowly evolving positions are less prone to confounding factors such as multiple substitutions, they probably retain an ancient phylogenetic signal6. Phylogenies constructed by examining these positions are less affected by artefacts. We used the 'slow–fast' method7 to identify these positions, which has proved efficient in analysing eukaryotic phylogeny based on rRNA5.
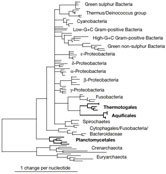
There are several discrepancies between the standard phylogeny2 and the one we have deduced using the most conserved positions (Fig. 1). Our version shows hyperthermophilic Bacteria (Aquificales and Thermotogales) to be monophyletic, which is consistent with large-scale studies3, 4, instead of paraphyletic as in classical versions. More important, our phylogeny shows the late emergence of hyperthermophiles, as they are clustered with Fusobacteria within a vast multifurcation containing almost all the phyla.
 |
Figure 1
Prokaryotic phylogenetic tree based on conserved positions in ribosomal
RNA. High resolution image and legend (53k) |
Surprisingly, Planctomycetales emerge at the base of the Bacteria. Whereas the support for the early branching of hyperthermophilic Bacteria is low (bootstrap value about 20%), slowly evolving positions (Fig. 1) provide reasonable, albeit inconclusive, support for the early emergence of Planctomycetales (bootstrap value about 70%). The basal position of hyperthermophilic phyla is found only when examining noisy (that is, fast-evolving) positions, and is thus probably artefactual. The artefactual attraction of the long branches8 of Archaea and hyperthermophilic Bacteria, and the high guanine and cytosine content of rRNA in most Archaea, Aquificales and Thermotogales9, could explain why hyperthermophiles are often found to branch early in Bacteria.
The emergence of hyperthermophilic Bacteria among mesophiles suggests a secondary adaptation to life at very high temperature for these organisms, which may have been facilitated by massive gene transfer from the Archaea10. The most convincing support for this idea is provided by the enzyme reverse gyrase, which is specific to hyperthermophiles11. This enzyme, which introduces positive supercoils in DNA and is thought to stabilize it at high temperatures, has been independently acquired by Aquifex and Thermotoga from Archaea11. Consistent with the ancestral guanine and cytosine content of rRNA9, our results indicate that the most recent common ancestor of Bacteria was not hyperthermophilic.
We find that Planctomycetales are the first branching bacterial group (Fig. 1). This is a significant, although understudied, division of Bacteria, whose members share several peculiar traits, such as a budding mode of reproduction and the lack of peptidoglycan in their cell walls12. The most intriguing feature of this group is the existence of a single or double membrane around the chromosome in Gemmata sp. and Pirellula sp., respectively, which has been compared to the eukaryotic nucleus12. However, evolutionary homology of these structures with the eukaryotic nucleus has not been proved. An early emergence of Planctomycetales, as inferred from our work, must be confirmed by a careful phylogenetic analysis of genome sequences from several apparently early-branching Bacteria. If our finding is verified, the origin of Bacteria should be seriously reconsidered.
CÉLINE BROCHIER
AND HERVÉ PHILIPPE
Phylogénie,
Bioinformatique et Génome, UMR 7622 CNRS, Université Pierre et Marie Curie, 9
quai St Bernard, 75005 Paris, France
e-mail: herve.philippe@snv.jussieu.fr
| 1. | Stetter, K. O. Ciba Found. Symp. 202, 1-10 (1996). | ISI | |
| 2. | Pace, N. R. Science 276, 734-740 (1997). | ISI | |
| 3. | Brochier, C., Bapteste, E., Moreira, D. & Philippe, H. Trends Genet. 18, 1-5 (2002). | ISI | |
| 4. | Daubin, V. & Gouy, M. Genome Inform. Ser. Workshop Genome Inform. 12, 155-164 (2001). |
| 5. | Philippe, H., Germot, A. & Moreira, D. Curr. Opin. Genet. Dev. 10, 596-601 (2000). | ISI | |
| 6. | Felsenstein, J. J. Mol. Evol. 53, 447-555 (2001). | ISI | |
| 7. | Brinkmann, H. & Philippe, H. Mol. Biol. Evol. 16, 817-825 (1999). | ISI | |
| 8. | Felsenstein, J. Syst. Zool. 27, 401-410 (1978). | ISI | |
| 9. | Galtier, N., Tourasse, N. & Gouy, M. Science 283, 220-221 (1999). | ISI | |
| 10. | Nelson, K. E. et al. Nature 399, 323-329 (1999). | ISI | |
| 11. | Forterre, P., Bouthier de la Tour, C., Philippe, H. & Duguet, M. Trends Genet. 16, 152-154 (2000). | ISI | |
| 12. | Fuerst, J. A. Microbiology 141, 1493-1506 (1995). | ISI | |