

Soils, Weathering, and Nutrients
From our previous discourse we know that all chemical elements, with the exception of
hydrogen and helium, came from the dying throes of large stars. Furthermore, we know that
the amino acids needed to build animal proteins come from autotrophic plant life.
Biological studies have shown how certain nutrients (e.g., nitrogen, phosphorus, calcium,
magnesium, potassium, iron, etc.) are essential for the process of protein formation. All
our amino acids and nutrients eventually come to us from plant life (sometimes via the
meat of plant-eating animals). Plants synthesize amino acids from the combination of
sunlight, water and soils.
Weathering
Since early geologic time, the atmosphere has interacted with the Earth's exposed crust
though a process known as weathering. Weathering takes place through a combination of both
mechanical and chemical means. We have all experienced the results of weathering first
hand. Any visit to an old cemetery find us peering at the blurred inscriptions on old
marble tombstones. These inscriptions were once perfectly legible, but with the passage of
time, the small fractures and cracks in the rock have made it vulnerable to attack by
aqueous solutions.
 |
 |
A dramatic example of weathering can be seen in these two images of the same 3000 year old Egyptian obelisk just before relocation to damp New York (at left) and 100 years after accelerated weathering in New York (at right). The obelsik had survived quite well in the dry climate of Egypt, but deteriorated rapidly in the humid and polluted (acids) air of New York. Weathering rates are obviously a strong function of climate. |
Many of the original volcanic gases (e.g., carbon dioxide, sulphur-bearing gases, etc.) that formed the early atmosphere, were able to dissolve in water and produce acids. These acids, in turn, reacted with surface minerals. Later, oxygen in the atmosphere reacted with the exposed reduced materials, making the red beds discussed in an earlier lecture. Since the advent of land plants, soil and surface minerals have been exposed to relatively high concentrations of carbon dioxide maintained in soil pores as a result of decomposition and the metabolic activities of roots. The reaction of carbon dioxide with water in the soil produces carbonic acid (H2CO3) which determines the rate of rock weathering in most ecosystems.
CO2 (gas) + H2O (liquid) --> H2CO3 (solution)
Acid rain, produced by human effluents of nitrogen and sulphur-bearing gases will increase
the rate of rock weathering in downwind areas. To understand why weathering occurs and why
the rate of rock weathering is so dependent on climate, we need to discuss the chemistry
of the process in a little more detail.
The result of the chemical breakdown of the original igneous rocks can be summarized as
follows:
"Igneous Rocks + Acid Volatiles = Sedimentary Rocks + Salty Oceans"
What we mean by this will become clearer if we look at the details of the weathering
processes. Consider a boulder or rock containing Feldspars, the most common group of
minerals in the Earth's crust. Feldspars are weathered through a chemical process
called hydrolysis, and it works in principle as outlined in the following equation:
2K Al Si3O8 + H2O+H2CO3
--> K2CO3+Al2Si2O5(OH)+4SiO2aqu
In this chemical formula feldspar reacts with carbonic acid and water to form potassium
carbonate (dissolved in water), kaolinite (a clay mineral, a solid), and dissolved silica
(denoted by the subscript aqu). Thus, during the hydrolysis reaction, soluble
elements (potassium, silica) are leached out of the weathering horizon (soil), an
alteration product is left behind (kaolinite), and acid is consumed (neutralized).
In this way, rocks containing feldspars are weakened though the conversion of
rigid feldspar to more plastic clays which do not have anything like the same structural
rigidity. The process occurs at exposed surfaces of the minerals making up the rock. The
debris of weathering processes is the source material for all sedimentary rocks, and
through geologic time large amounts of sedimentary rocks have been deposited as part of
this process.
Rock weathering is critical for the release of biochemical elements that have no gaseous
form - examples are calcium (Ca), Potassium (K), Iron (Fe), and Phosphorus (P). The latter
element plays a key role in cell metabolism. Thus, we can say that weathering provides key
nutrients for life through chemical breakdown of minerals in crustal rocks.
Chemical weathering reactions depend on a supply of acid, and in early Earth history
the higher CO2 content of the air may have helped to have sufficiently acid
rain to drive the process forward. Today, however, the CO2 content is so
low that the acidity of rainwater itself would fast be exhausted and neutralized.
The picture changes, however, when the land surface is colonized by organisms.
Today, for example, the roots of plants and soil bacteria produce abundant CO2
through their respirative and metabolic processes, and it this constant resupply of
CO2 in combination with moisture that leads to formation of more acid and keeps
hydrolysis humming along. In the Proterozoic, colonization of the land surface by
cyanobacterial communities may have served a similar purpose.
Soil
The addition of life the the weathering surface layer is of critical importance.
This combination of weathering blanket and life is what we call a soil, a dynamic
natural body capable of supporting a vegetative cover. Today, where there is no soil,
there is no plant life and we have barren rock and/or sand. Soil is composed primarily of
weathered materials, along with water, oxygen and organic materials.
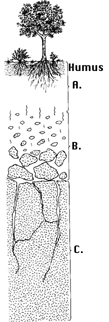 |
Soil is layered into sections called "horizons". The figure at left shows a typical soil profile developed on granite bedrock in a temperate region. The top horizon is composed of humus and contains most of the organic matter. This layer is often the darkest. The "A" horizon consists of tiny particles of decayed leaves, twigs and animal remains. The minerals in the A-horizon are mostly clays and other insoluble minerals. Minerals that dissolve in water are found at greater depths. The "B" horizon has relatively little organic material, but contains the soluble materials that are leached downwards from above. The "C" horizon is slightly broken-up bedrock, typically found 1-10 meters below the surface. While this is a typical soil profile, many other types exist, depending on climate, local rock conditions and the community of organisms living nearby. The U.S. Department of Agriculture has classified 10 orders and 47 suborders of soils. If you include other subsets, there are over 60,000 types of soil. The lunar surface, which has been produced by meteoroid impacts, is not classified as a soil, but is rather given the name "regolith" (derived from the Greek words meaning cover and stone). The layered nature of soil indicates its long evolution under the effects of atmospheric and biological processes. |
Nutrients
Although living tissue is composed of carbon, hydrogen, and oxygen in the
approximate proportion of formaldehyde (CH2O), as many as 23 other elements are necessary
for biochemical reactions and for the growth of structural biomass.
Examples of important nutrients are:
| Nutrient | Role |
| Nitrogen | the proteins found in plants and animals contain about 16% by weight of nitrogen |
| Phosphorus | P is part of the important ribulose biphosphate carboxylase molecule and is
part of ATP - adenosine triphosphate, the universal molecule for energy transformations |
| Calcium | Ca is a major structural component of the proteins forming plants and animals |
Other important nutrients include magnesium, potassium, iron, sulphur, etc. We should
note that, although carbon, nitrogen and sulphur can be obtained from the atmosphere,
calcium, magnesium, potassium, iron, and phosphorus all come from rock weathering
processes. The atmosphere has no store of these essential nutrients. As mentioned
earlier, one of the main purpose of plant root systems is to get access to nutrients
stored in the soil. Some plants go to enormous lengths to do this. For example, Emiliani
(1992) quotes that "A single plant of winter rye, 50 cm high, was found to have a
root system consisting of 143 main roots, 35,600 secondary roots, 2.3 million tertiary
roots, and 11.5 quaternary roots! The root system was found to have a total length of 600
km and a total surface of about 250 square meters".
Delivery of nutrients into plant root systems can occur by several pathways. In some
cases, direct uptake in water solution occurs. Sometimes, plants actually have to protect
themselves against too much nutrient intake. Too much of a good thing can prove poisonous.
An example of this can be seen in the accumulations of calcium carbonate deposits that
surround the roots of some desert shrubs.
| Some nutrients, such as nitrogen, phosphorus, and potassium are often harder for roots to find and specialized (incredibly efficient) enzymes have evolved. These are located in root membranes to seek out these scarce and needed resources. If some nutrients are not readily available, plants will grow more slowly and/or increase their root/shoot ratio. The approximate ratio of needed nutrients is shown in the table to the right. |
|
In general, the availability of nutrients (deficit or surplus availability) often controls
the form of the ecosystem, limiting the ability of a particular ecological niche to
support a particular set of plants. Excess nitrogen, for example, can lead to the loss of
fine root biomass and deficiencies in other nutrients. The pool of nutrients held in the
soil and vegetation is many times larger than the annual receipt of nutrients from the
atmosphere and rock weathering. Much of the nutrients needed to support plant life
are stored up in the humus of soils.
| Percentage of Annual Nutrient Requirement for Growth of Hardwood Forest | |||||
| Process (kg/ha/yr) | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| Total Growth Requirement | 115 | 12 | 67 | 62 | 10 |
| Atmospheric Inputs | 18 | 0 | 1 | 4 | 6 |
| Rock Weathering Inputs | 0 | 13 | 11 | 34 | 37 |
| Reabsorptions (Intra-system) | 31 | 28 | 4 | 0 | 2 |
| Detritus Turnover | 69 | 81 | 86 | 85 | 87 |
The data of the table above came from a study of the famous Hubbard Brook ecosystem in
New Hampshire. It shows how effective the soils are in storing the needed nutrients
(detritus turnover and readsorption). Acid rain caused by human emissions of
nitrogen and sulphur oxides leads to enhanced weathering and changes in nutrient ratios.
For example, recent studies suggest that forest growth has declined in areas downwind of
air pollution. Acid rain appears to increase the movement of aluminum ions, which may, in
turn, reduce the intake rates of calcium and other nutrients.
In the oceans, life is also limited by the availability of nutrients. The productivity is
highest on continental shelves and in regions of upwelling. Nutrients are removed from
surface waters by downward sinking and are regenerated in deep waters.